Table of Contents
Are CPAP Machines Approved by FDA
 The FDA issued a comprehensive emergency policy allowing alternative devices to be used as potentially lifesaving ventilators as shortages start to affect hospitals’ responses to the coronavirus pandemic.
The FDA issued a comprehensive emergency policy allowing alternative devices to be used as potentially lifesaving ventilators as shortages start to affect hospitals’ responses to the coronavirus pandemic.
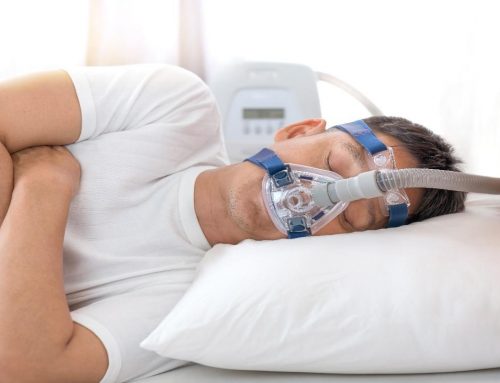
This includes modified anesthesia gas hardware and positive-pressure breathing devices—and home continuous positive airway pressure, or CPAP, devices used to treat sleep apnea, CPAP cleaners such as Soclean plus portable oxygen generators for chronic obstructive pulmonary disease and nasal cannulae tubes.
Nevertheless, the American Society of Anesthesiologists put out guidance recommending that healthcare specialists take extra precautions when utilizing such non-invasive respiratory devices, which may disperse unfiltered droplets and increase the spread of infection through the air.
The agency’s broad Emergency Use Authorization allows its use for treating COVID-19 patients following a manufacturer’s official request, and the FDA will be compiling a public list of permitted machines. Additionally, the FDA told healthcare providers that specific ventilators might be able to support multiple patients at once using air tube splitters.
 Earlier this week, the FDA told the industry it would permit manufacturers to modify and deploy previously cleared ventilators without requiring to resubmit them for agency review and permitted providers to utilize ventilators beyond their indicated shelf life.
Earlier this week, the FDA told the industry it would permit manufacturers to modify and deploy previously cleared ventilators without requiring to resubmit them for agency review and permitted providers to utilize ventilators beyond their indicated shelf life.
In the meantime, Medtronic and GE Healthcare are working to ramp up ventilator production. Medtronic, the Puritan Bennett brand maker, said it increased global output by more than 40% and intends to double its total capacity more than. GE Healthcare said it is setting up additional manufacturing lines, increasing shifts, hiring new workers, and boosting CT scanners, ultrasound devices, mobile X-rays, and patient monitors.




 Shop
Shop